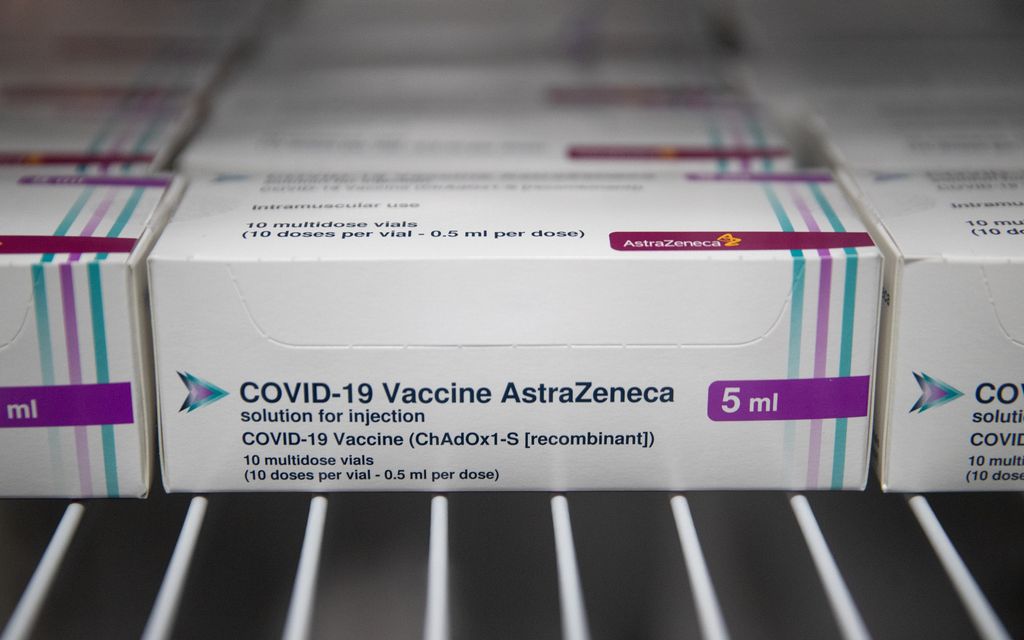
The European Union is expected to give the green-light to AstraZeneca’s Covid-19 vaccine on Friday, with the company under immense pressure to rectify anticipated delivery delays.
The European Medicines Agency (EMA) is anticipated to recommend the British-Swedish firm’s drug for conditional market authorization.
The European Commission could then give it formal approval within hours, making it the third shot at the bloc’s disposal, along with those made by German-US venture Pfizer-BioNTech and and US firm Moderna.
It is thought EMA might recommend the drug for use only for younger people at first due to relevantly scant data on its efficacy in over-65s. Germany’s top vaccine committee announced a similar move on Thursday.
Regardless of who could get the jab, the EU would still have to contend with significantly smaller first deliveries than promised.
AstraZeneca announced major hold-ups at an EU production site last week to the consternation of the commission and member states.
A series of crisis talks have failed to resolve the dispute so far.
In the meantime, the commission is likely to officially recommend on Friday the establishment of an EU “transparency register” on exports of Covid-19 shots outside the bloc until the end of March.
This would also allow blockades on exports of vaccines made in the bloc that the EU feels it is legally entitled to by its agreements with pharmaceutical firms, according to EU officials.







































admin in: How the Muslim Brotherhood betrayed Saudi Arabia?
Great article with insight ...
https://www.viagrapascherfr.com/achat-sildenafil-pfizer-tarif/ in: Cross-region cooperation between anti-terrorism agencies needed
Hello there, just became aware of your blog through Google, and found ...